Get treated by the pioneer of Online treatment, who was first to start Online Medical practice in 1995. In a few clicks from now, you can get started with the journey to recovery.

Send your query to get genuine homeopathy opinion. We have answered over a million people.
Dr. Asher's expert, research based treatments for major diseases. You will find extensive information on clicking following links.
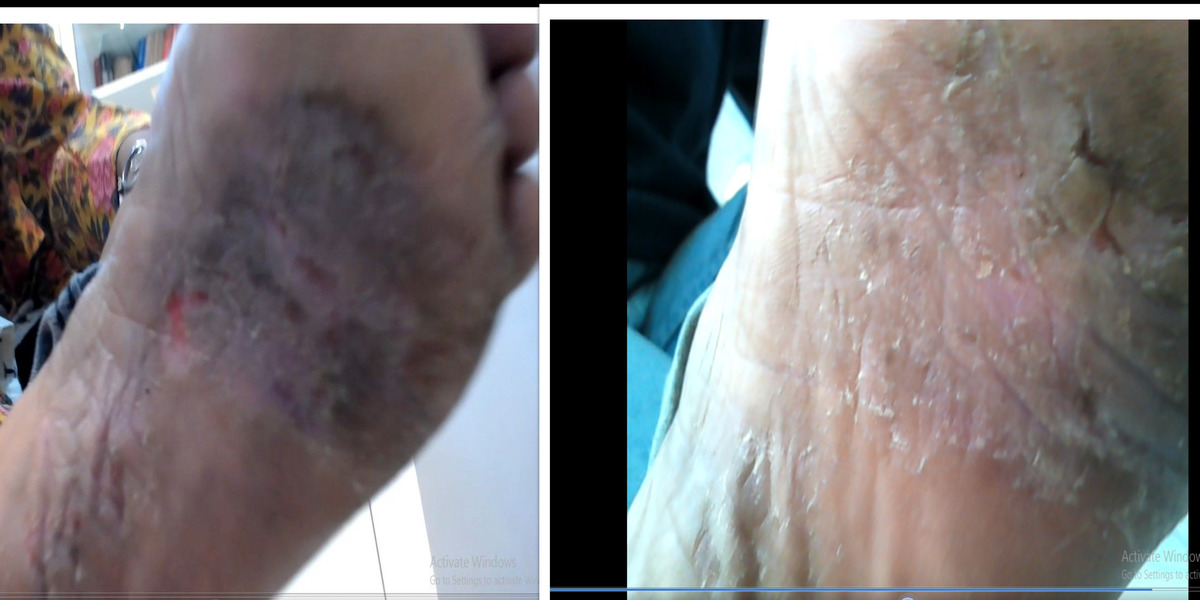
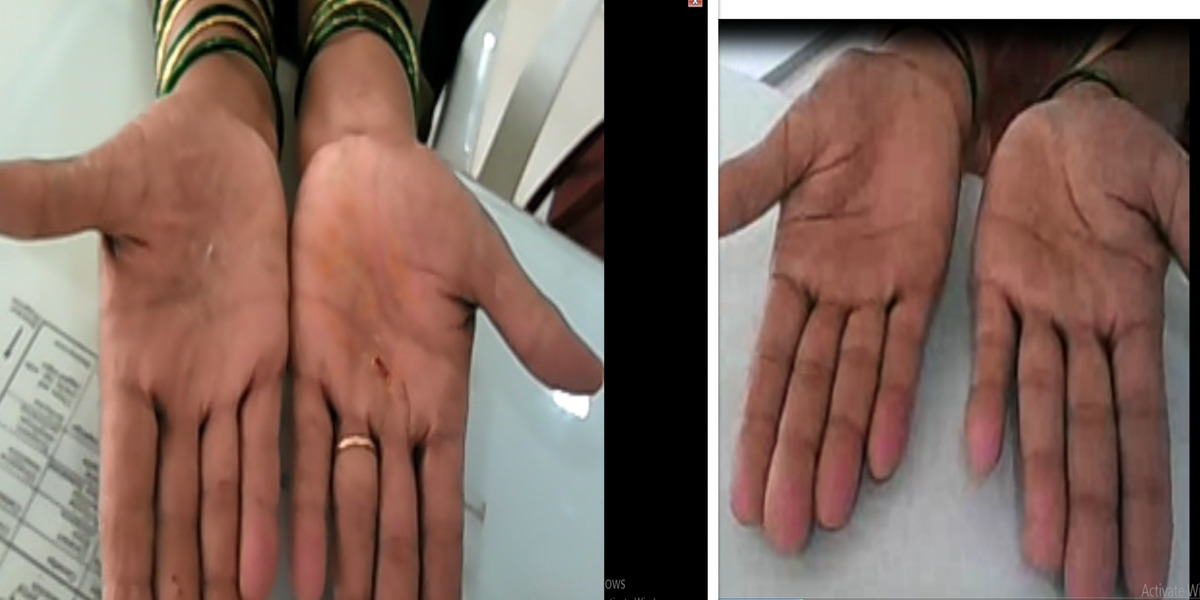
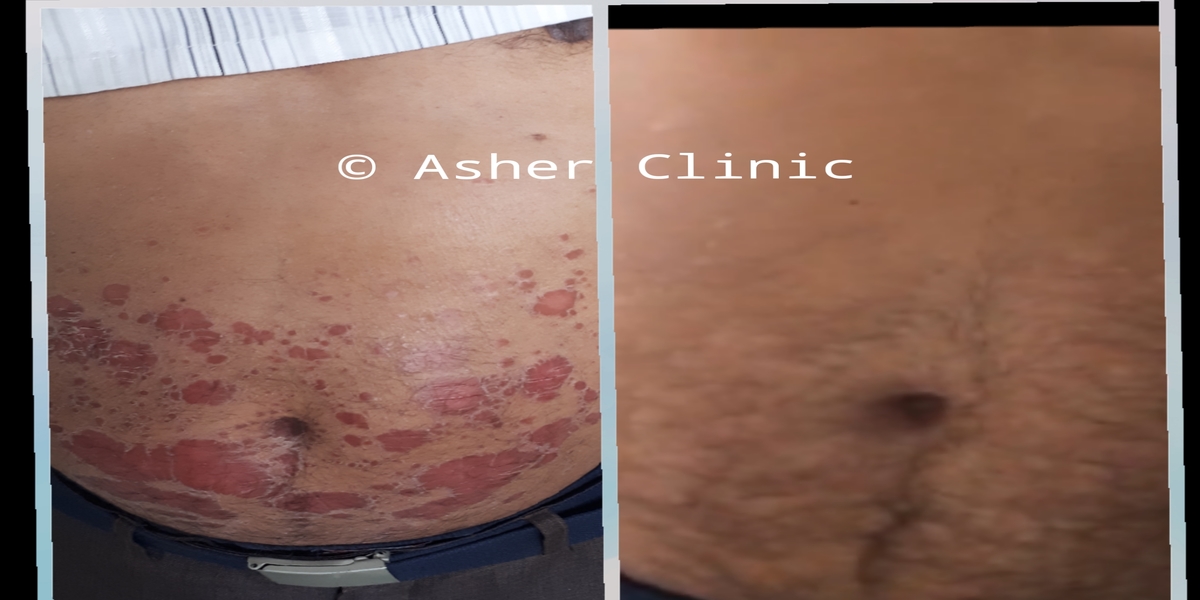
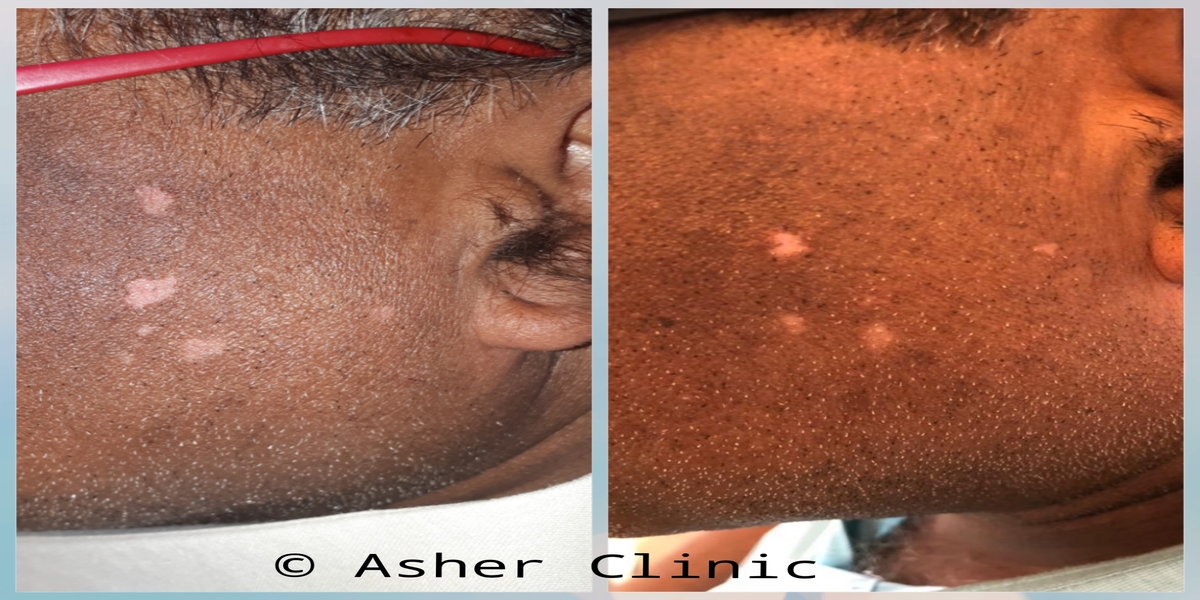
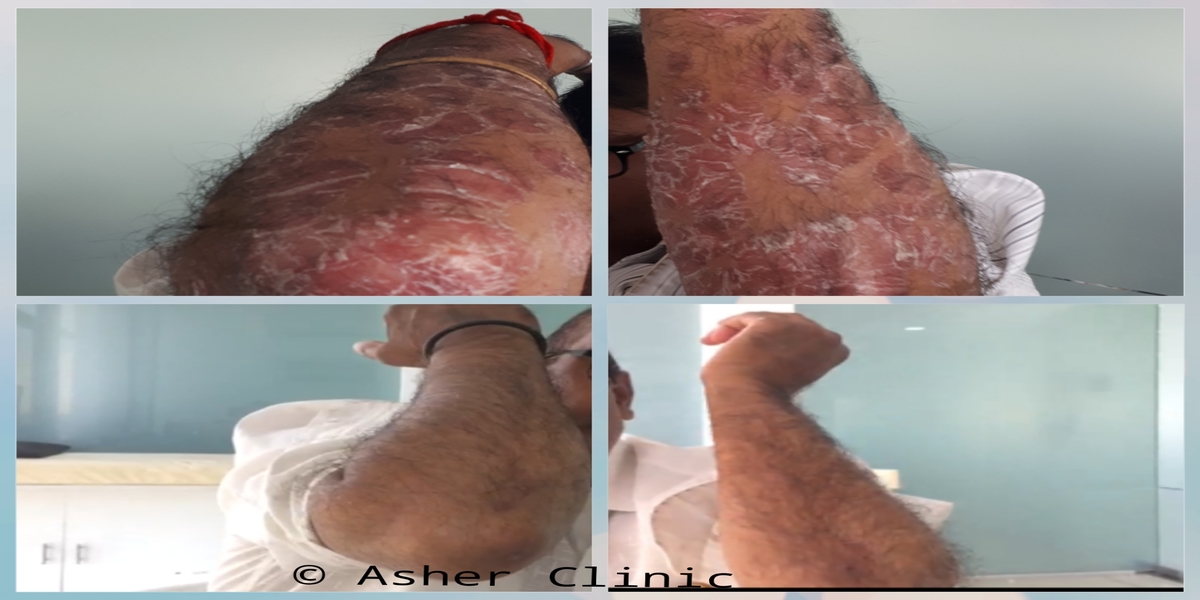
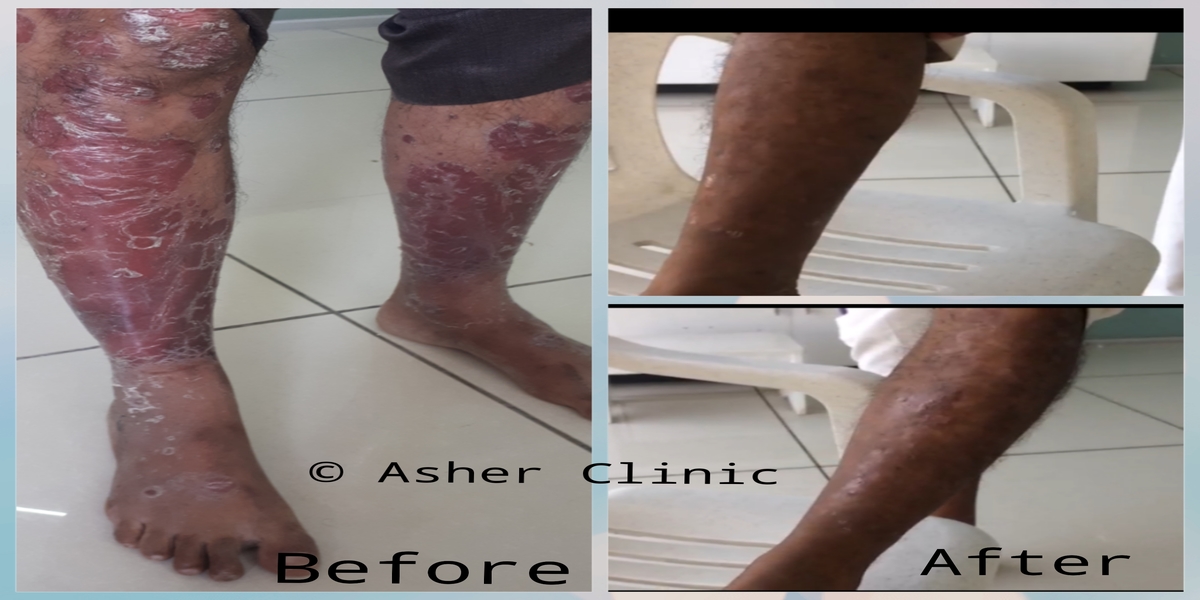
Patients from all across the world approach. Dr. Asher for the treatment of chronic and recurring diseases. Obviously, there are good doctors in all countries. Patients from many countries still contacted. Dr. Asher because they did not get well, due to the very nature of those difficult-to-treat-diseases. Those diseases included all kind of chronic diseases such as psoriasis, migraine, asthma, eczema, lichen planus, vitiligo, urticaria, trigeminal neuralgia, ulcerative colitis and many more.
Read More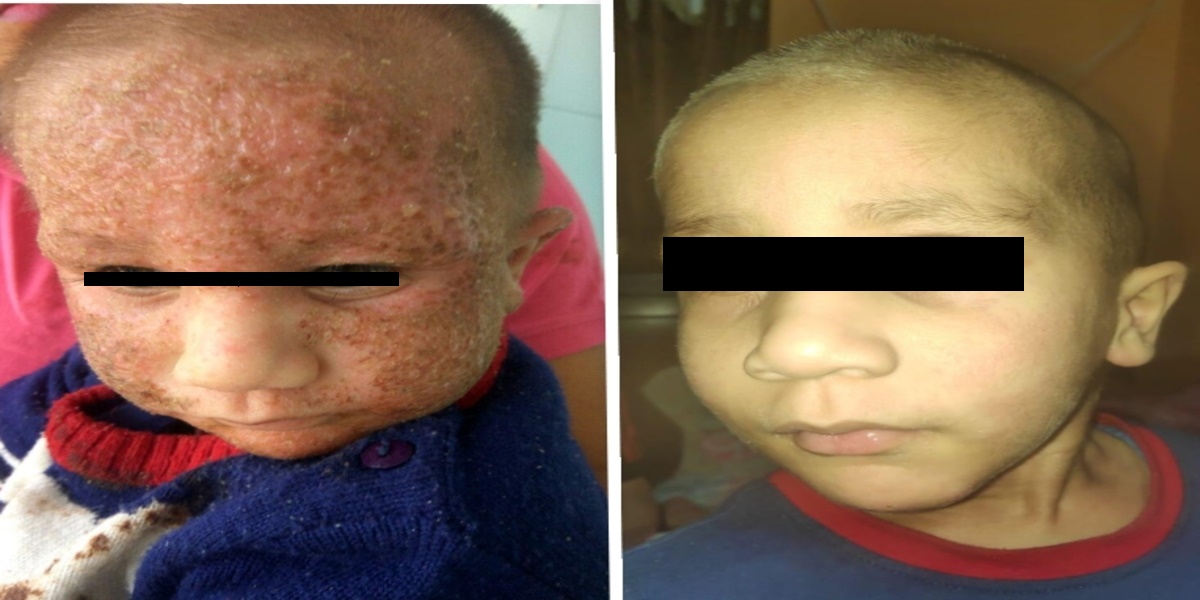
Copyright © 2017 asherclinic.com All rights reserved. Designed By
